Cancer Antigen Presenting Fibroblasts
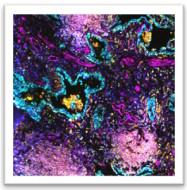 One of the most challenging questions in cancer immunotherapy is how we can sustain anti-tumor T cells within tumors. We recently discovered cancer antigen presenting fibroblasts and showed that they directly stimulate cancer-specific CD4 T cells, creating immunological hot spots that support immune rejection. We use computational and laboratory experiments and work in parallel in human and mouse models to understand the heterogeneity, configurations and the functions of antigen presenting cancer-associated fibroblasts. To dissect the molecular landscape that regulates fibroblast states we generate in vitro and in vivo perturbation datasets. We work to understand how diverse fibroblast states emerge and evolve and to functionally explain their role in cancer immunity. Our research decodes the complexity of antigen presentation in tumors and promotes targeting of the stroma for immunotherapy.
One of the most challenging questions in cancer immunotherapy is how we can sustain anti-tumor T cells within tumors. We recently discovered cancer antigen presenting fibroblasts and showed that they directly stimulate cancer-specific CD4 T cells, creating immunological hot spots that support immune rejection. We use computational and laboratory experiments and work in parallel in human and mouse models to understand the heterogeneity, configurations and the functions of antigen presenting cancer-associated fibroblasts. To dissect the molecular landscape that regulates fibroblast states we generate in vitro and in vivo perturbation datasets. We work to understand how diverse fibroblast states emerge and evolve and to functionally explain their role in cancer immunity. Our research decodes the complexity of antigen presentation in tumors and promotes targeting of the stroma for immunotherapy.
Dendritic Cells Type 1 in Cancer
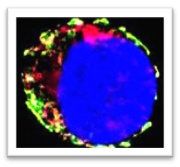 Dendritic cells type 1 (DC1) capture cancer antigens in tumors and migrate to lymph nodes, where they are uniquely capable of cross-presenting antigens to CD8 T naïve cells. However, they are scarce within tumor tissues, which restricts T cell priming, limits the effectiveness of immunotherapy, and negatively impacts patient outcomes. We recently described a mechanism by which type I interferons induce DC1 death within tumors. We work to further enhance our understanding of DC1 biology in cancer, including migration, survival and T cell stimulation. To achieve this, we deploy mouse models of cancer, alongside multiparameter spectral flow cytometry, high resolution imaging, in vitro and in vivo gene editing. We hope to translate our findings into new immunotherapies that will enhance DC1 and boost anti-tumor immunity.
Dendritic cells type 1 (DC1) capture cancer antigens in tumors and migrate to lymph nodes, where they are uniquely capable of cross-presenting antigens to CD8 T naïve cells. However, they are scarce within tumor tissues, which restricts T cell priming, limits the effectiveness of immunotherapy, and negatively impacts patient outcomes. We recently described a mechanism by which type I interferons induce DC1 death within tumors. We work to further enhance our understanding of DC1 biology in cancer, including migration, survival and T cell stimulation. To achieve this, we deploy mouse models of cancer, alongside multiparameter spectral flow cytometry, high resolution imaging, in vitro and in vivo gene editing. We hope to translate our findings into new immunotherapies that will enhance DC1 and boost anti-tumor immunity.
Lung Tumor Innervation
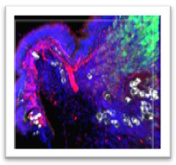 Emerging evidence suggest a link between tumor innervation and cancer progression, with implications for metastasis and recurrence in various types of cancer. The mechanisms by which existing nerves infiltrate or new ones arise within the tumor microenvironment remain unclear but are thought to involve complex interactions between tumor cells and the surrounding stroma. Our high-resolution spatial phenotyping and neuroimaging pointed out specific cellular and molecular networks that may drive lung tumor innervation. We are currently validating our observations and using them to generate novel mechanistic hypotheses on the emergence and evolution of tumor innervation. We aim to identify novel targets that will allow us to manipulate innervation for cancer therapy.
Emerging evidence suggest a link between tumor innervation and cancer progression, with implications for metastasis and recurrence in various types of cancer. The mechanisms by which existing nerves infiltrate or new ones arise within the tumor microenvironment remain unclear but are thought to involve complex interactions between tumor cells and the surrounding stroma. Our high-resolution spatial phenotyping and neuroimaging pointed out specific cellular and molecular networks that may drive lung tumor innervation. We are currently validating our observations and using them to generate novel mechanistic hypotheses on the emergence and evolution of tumor innervation. We aim to identify novel targets that will allow us to manipulate innervation for cancer therapy.
Organs-On-Chip
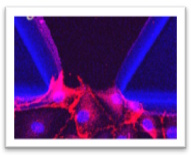 We focus on addressing the challenges in understanding human immune responses to cancer and infections, as well as the limitations of traditional in vitro models in simulating these interactions. The goal is to advance research by incorporating lung-on-chip technology, which mimics complex lung functions and interactions, offering a more accurate representation of physiological responses. The approach involves integrating human lung tissues into the lung-on-chip platform to study immune cell behavior, providing deeper insights into tumor/pathogen-host interactions and supporting the development of more effective and personalized therapeutic approaches.
We focus on addressing the challenges in understanding human immune responses to cancer and infections, as well as the limitations of traditional in vitro models in simulating these interactions. The goal is to advance research by incorporating lung-on-chip technology, which mimics complex lung functions and interactions, offering a more accurate representation of physiological responses. The approach involves integrating human lung tissues into the lung-on-chip platform to study immune cell behavior, providing deeper insights into tumor/pathogen-host interactions and supporting the development of more effective and personalized therapeutic approaches.
Omics Technologies
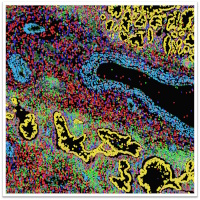 To phenotype our tissue and culture systems in detail we use a comprehensive array of advanced state-of-the-art omics technologies. Single-cell RNA sequencing, followed by clustering and dimensionality reduction, infers cellular heterogeneity and pinpoints cell types and states. To understand gene expression control we perform gene regulatory network analysis. Trajectory inference enables us to map developmental processes and trace cellular transitions, getting insights into differentiation and lineage pathways. We deploy spatial transcriptomics and highly multiplexed tissue imaging to record gene and protein expression at high spatial resolution.
To phenotype our tissue and culture systems in detail we use a comprehensive array of advanced state-of-the-art omics technologies. Single-cell RNA sequencing, followed by clustering and dimensionality reduction, infers cellular heterogeneity and pinpoints cell types and states. To understand gene expression control we perform gene regulatory network analysis. Trajectory inference enables us to map developmental processes and trace cellular transitions, getting insights into differentiation and lineage pathways. We deploy spatial transcriptomics and highly multiplexed tissue imaging to record gene and protein expression at high spatial resolution.