Elucidating the homeostatic mechanisms that safeguard tissue quality and organ size remains a major challenge in biology, necessary to acquire a deeper understanding of animal development and treat diseases.
Cell competition is one such quality control mechanism, where alterations in cellular properties are sensed non-autonomously in mosaic tissues, leading to the selective elimination of the “less-fit” population. Cell competition has been shown to be important to tissue homeostasis in both flies and mammals, acting as a quality control mechanism, optimizing tissue and organ fitness by eliminating suboptimal cells during development and adult life. Cell competition has also been involved in regeneration, aging and tumorigenesis. However, the mechanisms that determine cellular fitness and trigger competition remain elusive.
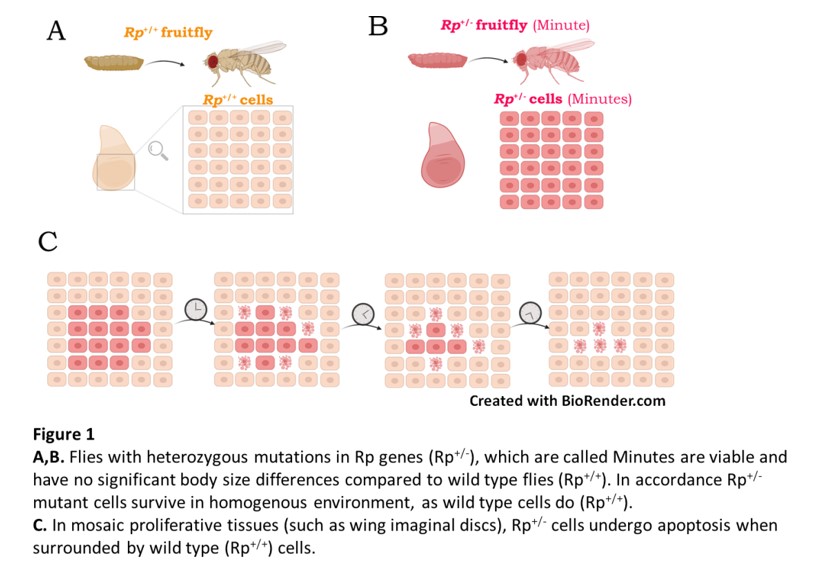
Cell competition was originally described in Drosophila, through the study of a class of dominant mutations in ribosomal protein (Rp) genes. In mosaic Drosophila tissues, cells heterozygous for mutations in ribosomal protein genes (Rp+/- cells) were selectively eliminated by wild-type cells. During my postdoctoral studies in the laboratory of Prof. Nicholas Baker, we identified the Xrp1 transcription factor, as the master regulator of the stress response pathway triggered in Rp+/- cells. We showed that Xrp1 controls most of the Rp+/- phenotypes, including the transcriptional changes, translation, growth, and cell competition. Recently, we showed that cells with multiple defects in ribosome biogenesis or function are eliminated by Xrp1 dependent cell competition. Interestingly, we found that Xrp1, in addition to single-copy genes, regulates multiple transposable elements (TEs) in Rp+/- cells. TEs are increasingly recognized to be involved not only in genome evolution, but also in various diseases.
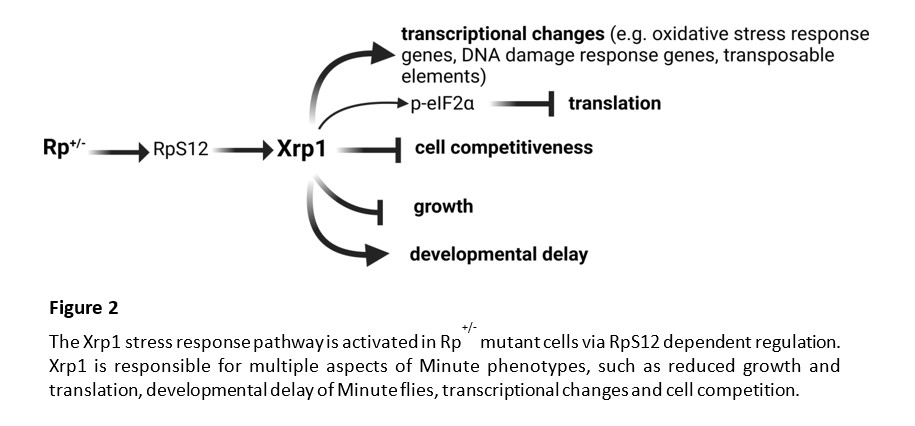
Heterozygous Rp+/- mutant flies (called Minutes) are viable, with near-normal size, although they present slower developmental rates, shorter bristles, poor fertility and reduced survival. Interestingly, heterozygous Rp mutations in humans have been identified as causal of Diamond Blackfan Anemia (DBA), an inherited bone marrow failure syndrome, characterized also by short stature, delayed maturation and skeletal defects. DBA belongs to the broader class of ribosomopathies, which are diseases linked to defects in ribosome biogenesis and function. Paradoxically, ribosomopathy patients present higher risk of cancer later in life and mutations in Rp genes promote cancer. There is a potential link between p53 in mammalian Ribosomopathies and Xrp1 in Drosophila Minutes. Also, given the evolutionary conservation of the ribosome machinery, studying Rp+/- mutant responses in Drosophila, will also provide us with a better understanding of the stress responses in human ribosomopathies and cancer.
Our research aims to contribute to the following aspects of developmental biology:
- elucidating the mechanisms driving cell competition
- elucidating the autonomous and non-autonomous stress responses upon ribosomal defects in proliferative and post-mitotic cells
- investigating the role of Transposable Elements in Ribosome mutant cells and in Cell Competition
We will apply genetic, transcriptomic, proteomic and pharmaceutical approaches in Drosophila to achieve our aims.
We are seeking for undergraduate and graduate students, with genuine interest in mechanisms that govern developmental biology, to join our team.