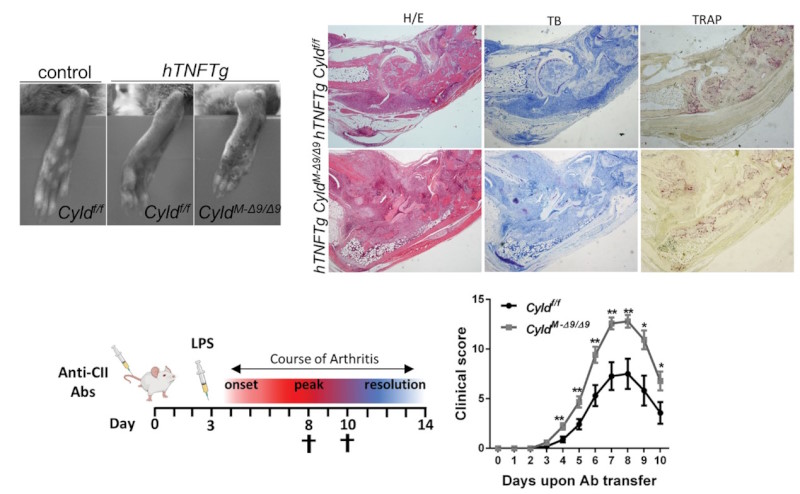
Research at the Armaka lab published in Cell Death and Disease identifies the deubiquitinase Cyld as a major checkpoint for the activity of synovial fibroblasts in inflammatory arthritis.
Cyld acts as a deubiquitinase that removes M1 and K63 ubiquitin chains to control survival and inflammation (NFkB, MAPK pathways) as well as death pathways. It was previously reported that Cyld is downregulated in Rheumatoid Arthritis Synovial Fibroblasts. However, the phenotypic and molecular consequences were poorly defined. By employing synovial fibroblast-specific genetic tools and mutant mice for Cyld, ikk2 kinase, Jnk2 and Mlkl, and TNF-mediated arthritic models, Rinotas, Iliaki et al revealed that Cyld limits K63 ubiquitination of TAK1 and uncontrolled NFkB activation in synovial fibroblasts -without interfering with their death responses, and therefore, restrains the progression of arthritis.
This study builds on previous knowledge of the lab and adds on the blueprint of important signaling partners that control the arthritogenic activity of synovial fibroblasts.
Rinotas V, Iliaki K, Pavlidi L, Meletakos T, Mosialos G, Armaka M*.
Cyld restrains the hyperactivation of synovial fibroblasts in inflammatory arthritis by regulating the TAK1/IKK2 signaling axis Cell Death Dis. 2024 Aug 9;15(8):584.
https://doi.org/10.1038/s41419-024-06966-2 PMID: 39122678
*Corresponding author